Measuring force for cellular shoving match
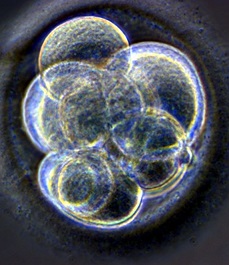 In the growing embryo, cells jostle for space by tugging and nudging their neighbours, and now there is a way to measure the tiny forces they exert on each other in the struggle for life.
In the growing embryo, cells jostle for space by tugging and nudging their neighbours, and now there is a way to measure the tiny forces they exert on each other in the struggle for life.
It may seem like cells shoving each other out of sheer spite or rudeness, but the microscopic manhandling does happen for a reason.
Studies in lab-grown cells have suggested that mechanical forces are as important in regulating biological function as chemicals and genes, but scientists had no way of quantifying the mechanical forces at specific positions in living tissues.
Biological tissues do not just float around waiting to grow up — they are constantly in motion, with cells pushing and pulling other cells and the extracellular matrix — the molecular scaffold that knits cells together into tissues.
As a result, tissues live in a state of dynamic tension, like a partially stretched rubber band.
The various cellular forces are particularly important as the body develops from the fertilised egg into tissues and organs with special shapes and functions — a process known as morphogenesis.
As the embryo develops, mechanical forces direct cells to multiply, steer to their proper locations, and specialize in particular ways.
“Shaping tissues and organs involves an interplay between genetics and physics. If you can't measure the physical side of it, you can't completely understand the problem,” said Otger Campàs, a professor of mechanical engineering and expert in systems biology.
So Campàs invented his own method; using microscopic droplets of oil as force transducers in living tissues.
Campàs and his colleague, Professor of Bioengineering Don Ingber, used a special oil called a fluorocarbon that remains separate from the cell membrane, like oil does from water, and is safe for cells and tissues.
A special coating was created for the droplets so they would stick to cells or to the extracellular matrix. This enabled them to measure how cells push and pull within living tissues.
The droplets were also set to light up under exposure to a laser, which would enable the deformations to be captured on film.
By measuring how deformed each droplet was, the scientists could precisely calculate the squeezing and stretching forces exerted by neighbouring cells and by the extracellular matrix.
Measurement of mouse mammary cells revealed that an individual cell can exert huge forces — 24 times as much pressure on the droplet as the jaws of an ant – and that the cells exerted the same amount of force in cultured aggregates as in tissues, suggesting the method is quite accurate.
Dr Campàs will now use the method to determine the patterns of force that shape different embryonic structures in fish, chicken, and other organisms.








 Print
Print