Liquid switch seen early
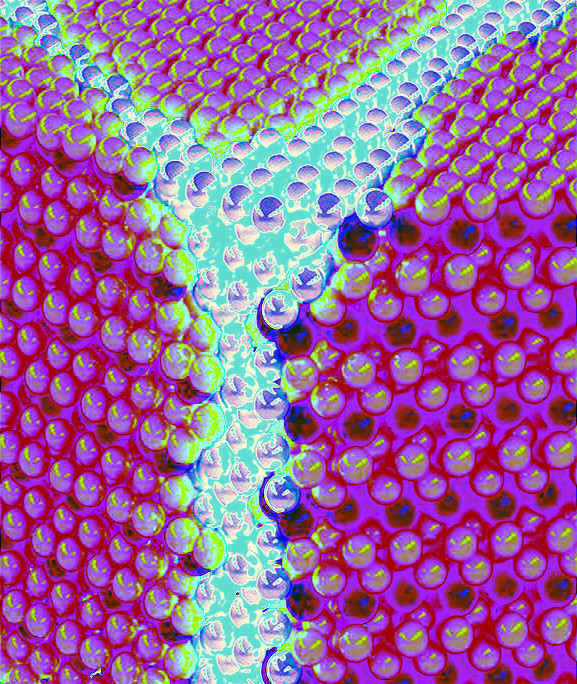 The boundary between solid and liquid metal is far more dynamic than previously known.
The boundary between solid and liquid metal is far more dynamic than previously known.
A new phenomenon, observed in metals like the copper-gallium alloy, shows the metal's surface oscillating between solid and liquid states at temperatures significantly below their typical melting points.
Scientists at RMIT University in Melbourne have uncovered a startling behaviour in metal surfaces, demonstrating that these can liquefy and then re-solidify in a fluctuating manner.
This phenomenon occurs unexpectedly at temperatures up to 200°C below the typical melting point of the metal involved.
“This fluctuation of the solid metal surface between solid and liquid phases was completely unexpected, especially since the entire system was kept at close to room-temperature conditions,” says lead researcher Caiden Parker, a PhD candidate with RMIT's Functional Materials and Microsystems Research Group.
The experimental observations were made using a Transmission Electron Microscope (TEM) which provided clear images of the metal's surface transitioning between phases at a nanometer scale.
The study reveals that solvent atoms at the surface of the solid metal begin to escape into the surrounding liquid metal, creating vacancies and destabilising the lattice structure.
This instability leads to the temporary liquefaction of the surface.
Subsequently, the surrounding liquid becomes enriched with solute atoms, causing it to recrystallise and solidify once more.
“This cyclic transformation of the alloy's surface could be a key to understanding the stability of certain alloys and the process of crystallisation in general,” said Professor Torben Daeneke, another lead researcher on the project.
The findings could have significant implications for the production and stabilisation of metal alloys used in various industrial applications, including manufacturing and 3D printing technologies.
As the alloying process is fundamental to many industries, this discovery opens new avenues for research into metal chemistry and alloy stability.
“We can't know yet what applications this might ultimately lead to. We don’t know whether someone will use this new understanding to synthesise improved alloys, or to reduce energy-use in alloy creation, or... who knows what! That’s why fundamental science is so cool,” Parker said.
The full study is accessible here.








 Print
Print